Revolutionizing Cancer Detection: A Label-Free Breakthrough That Lets Cancer Cells "Speak" for Ultra-Sensitive Monitoring of Cell Death and Trace Detection
Written & Image credit to Chen-Sheng Yeh
Early diagnosis is critical for improving cancer treatment outcomes. A research team led by Chair Professor Chen-Sheng Yeh of the Department of Chemistry at National Cheng Kung University (NCKU) has developed a groundbreaking cancer detection and treatment technology using gold nanoparticles encapsulated within electroactive liposomes. This innovative method eliminates the need for fluorescent dyes—laser light alone is enough to make cancer cells “speak.” By leveraging surface-enhanced Raman scattering (SERS) to amplify specific signals, this approach enables the ultra-sensitive detection of even trace amounts of cancer cells.
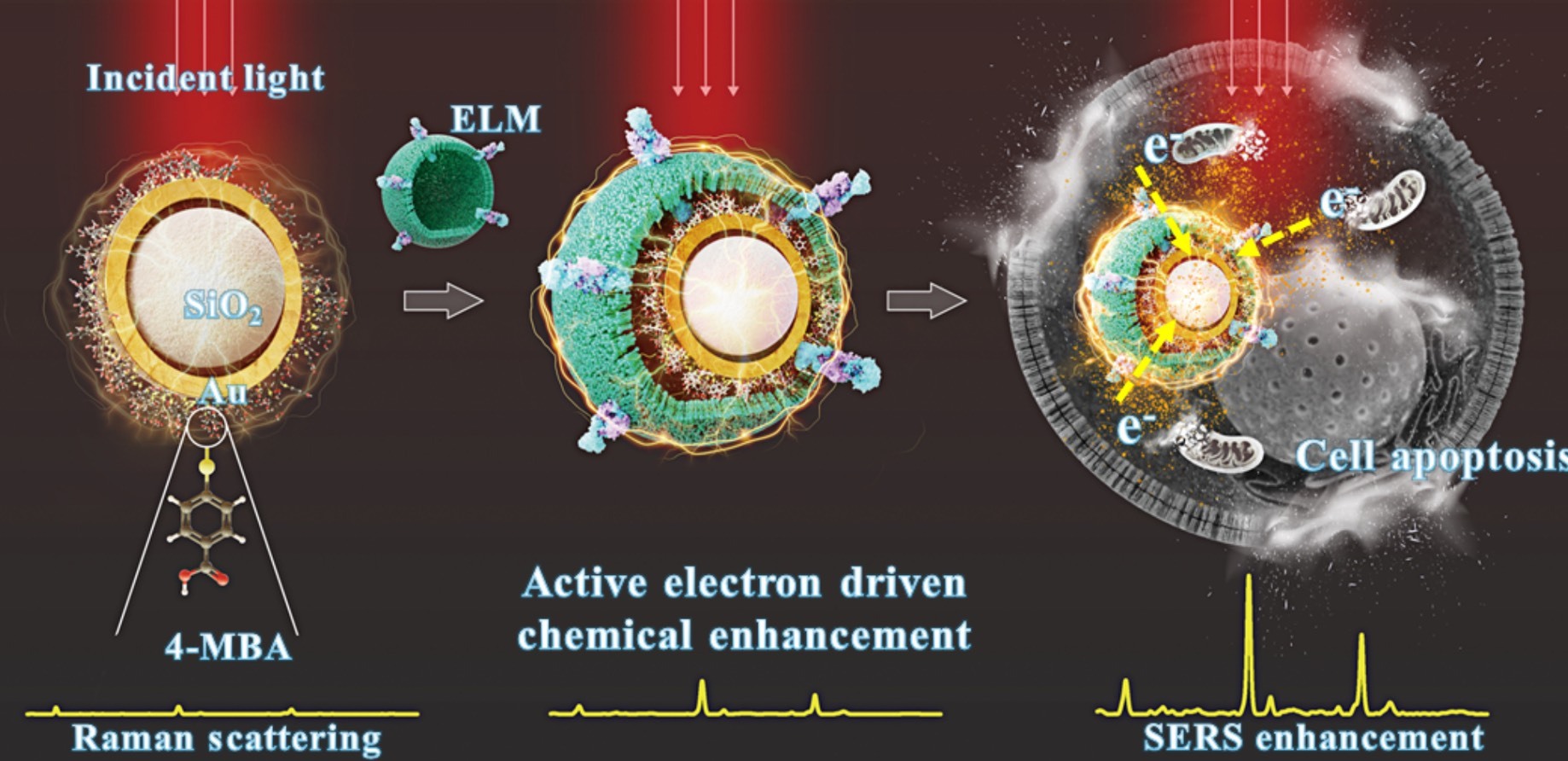
Chair Professor Chen-Sheng Yeh from the Department of Chemistry at National Cheng Kung University leads a team that has developed a method for accurate early cancer detection. The team coated gold nanoshells with electroactive liposomal membranes derived from Shewanella oneidensis, enabling the liposomes to actively transfer electrons—generated by cancer cell mitochondria—to the gold nanoparticles.
Remarkably, this technology not only identifies cancer cells but also initiates their destruction by disrupting their internal redox balance, ultimately inducing cell death. In experiments with liver cancer cells, over 90% were successfully eradicated, underscoring the platform’s immense therapeutic potential. The team is now collaborating with local biotech firms to commercialize the technology and accelerate its path to clinical application.
Professor Yeh explained that their pioneering “electron-driven cancer cell apoptosis and SERS” platform has recently been published in two top-tier journals: Advanced Materials and Nature Communications. Patents are being filed in both the United States and Taiwan, and a confidentiality agreement has been signed with Leadgene Biotech to jointly develop diagnostic products. As diagnostics are typically easier and faster to commercialize than cancer therapeutics—which require extensive clinical trials and greater investment—the initial focus is on detection tools. However, the team is also advancing therapeutic applications, including the development of an inhalable lung cancer treatment in collaboration with Dr. Chien-Chung Lin from the Division of Pulmonary and Critical Care at NCKU Hospital.
Redox disruption using electroactive liposome coated gold nanoparticles for cancer therapy
Utilizing Electron-Sink-Enhanced Nanoshells for Amplified Nanoplasmonic SERS-Based In Situ Detection of Cancer Cells, Linking Signal Enhancement with Cellular Damage
Redox disruption using electroactive liposome coated gold nanoparticles for cancer therapy
Utilizing Electron-Sink-Enhanced Nanoshells for Amplified Nanoplasmonic SERS-Based In Situ Detection of Cancer Cells, Linking Signal Enhancement with Cellular Damage

Chair Professor Chen-Sheng Yeh from the Department of Chemistry at National Cheng Kung University leads a team that has developed a gold nanoparticle-encapsulated electroactive liposome technology. Utilizing the principle of surface-enhanced Raman scattering (SERS), this method enables accurate early cancer detection. From left: research assistant Ming-Che Chang, Assistant Professor Hung-Kang Tian from the Department of Chemical Engineering, Professor Chen-Sheng Yeh, and research assistant Kai-Lin Chang.
These landmark achievements stem from a collaborative, interdisciplinary effort involving postdoctoral researchers Dr. Liu-Jun Wang and Dr. Ying-Chi Chen; Professor Wen-Pin Su, Director of the NCKU Institute of Clinical Medicine and Clinical Research Center, and his postdoc Dr. Li-Chan Chang; Professor Wen-Tai Chiu, NCKU Vice President for R&D and faculty in Biomedical Engineering; Assistant Professor Hung-Kang Tian from the Department of Chemical Engineering; Associate Professor Yi-Hsin Chien of the Department of Materials Science and Engineering at Feng Chia University; and Associate Professor Wei-Peng Lee from the School of Pharmacy at Kaohsiung Medical University.
Yeh emphasized the limitations of conventional cancer detection methods, such as antibody-based blood tests, contrast agent injections, and glucose isotope imaging, which often lack precision at early stages. In contrast, SERS enables the amplification of otherwise imperceptible signals, dramatically improving detection sensitivity and enabling this novel diagnostic strategy.
Cancer cells typically exhibit elevated redox activity and generate large volumes of electrons due to their heightened metabolic state. To harness this property, the team coated gold nanoparticles with electroactive liposomal membranes (ELMs) derived from the bacterium Shewanella oneidensis MR-1. These ELMs actively mediate electron transfer from cancer cells to the gold nanoparticles. When exposed to laser light, the captured electrons induce strong plasmonic resonance, significantly enhancing the Raman signal and enabling high-sensitivity detection.

Chair Professor Chen-Sheng Yeh from the Department of Chemistry at National Cheng Kung University leads a team that has developed a gold nanoparticle-encapsulated electoractive liposome technology. Utilizing the principle of surface-enhanced Raman scattering (SERS), this method enables accurate early cancer detection. In the photo, Professor Yeh (right) guides the team through experiments.
This technology is extraordinarily sensitive. Yeh noted that it could detect as few as five cancer cells. Unlike traditional methods, this system requires no fluorescent labeling—electron transfer occurs the moment a cancer cell interacts with the gold nanoparticle. Because cancer cells generate far more electron flow than normal cells, their Raman signals are vastly stronger, allowing for clear differentiation between healthy and malignant cells.
Normal cells exhibit only minimal electron transfer, and even after prolonged incubation, their signal remains far weaker than that of cancer cells. This demonstrates the system's superior precision in cancer detection. Uniquely, the technology also exhibits an inverse signal-to-cell ratio: the fewer the cancer cells present, the stronger the signal becomes. Furthermore, it can monitor cancer cell apoptosis, showing distinct signal intensities for different cancer types—liver cancer cells currently produce the strongest signal.
As Yeh described, the gold nanoparticles effectively "drain" cancer cells of electrons, cutting off their energy supply and causing them to shut down and die. In addition to eliminating over 90% of liver cancer cells, the method has also destroyed more than 80% of breast cancer cells in experimental settings. Large-animal (canine) trials have demonstrated significant tumor cell apoptosis after three weeks of treatment. The treated dogs maintained normal appetites and showed no major inflammation or discomfort—unlike the harsh side effects typical of chemotherapy—highlighting the method’s gentleness and potential for broader therapeutic use.
The team has signed a confidentiality agreement with Leadgene Biotech and is actively moving forward with commercialization plans. Their real-time, label-free, and ultra-sensitive cancer detection technology is expected to find applications in cancer diagnostics, biosensing, and medical imaging, advancing the development of personalized medicine. When paired with their “power cut-off” cancer therapy, it could usher in a new era in cancer treatment.
Provider:
NCKU News Center
Date:
2025-04-25
Click Num:
Share

