NCKU Research Team, Led by Prof. Win-Pin Su, Achieves Breakthrough in Lung Cancer Treatment: Published Study on Tumor Microenvironment Remodeling Using Nanotechnology in Prestigious Journal《 Nano Today 》
Written by NCKU Institute of Clinical Medicine. Image Credit to NCKU News Center
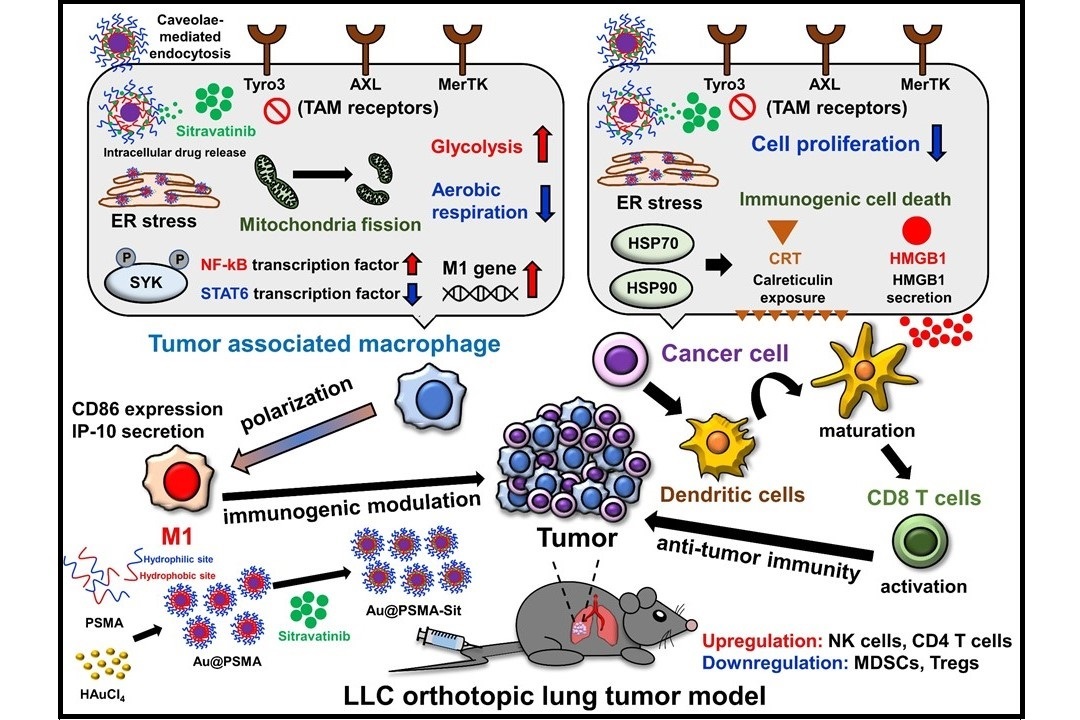
The Au@PSMA-Sit nano-drug enhances the anti-lung cancer efficacy by remodeling the tumor immune microenvironment (Nano Today. 2024, 54, 102070).
Tumor microenvironment (TME) primarily contributes to resistance in clinical antineoplastic treatment. In non-small-cell lung cancers (NSCLCs), the frequently mutated KRAS oncogene (mainly at the KRASG12C position) promotes tumor progression. The interdisciplinary research team at NCKU has successfully nano-formulated the small-molecular drug Sitravatinib (currently undergoing clinical trials internationally) using polymeric nanoparticles to form the novel nano-drug Au@PSMA-Sit. In the murine lung cancer model bearing KRASG12C mutant TME, Au@PSMA-Sit nanoparticles exhibit outstanding antitumor efficacy, requiring a lower therapeutic dosage (1/10) of sitravatinib. These experimental achievements were published in the authoritative international journal Nano Today in February 2024 with an impact factor of 17.4. Polymeric nano-formulation of spectrum selective RTK inhibitor strengthens anti-cancer effects via immune remodeling by endoplasmic reticulum stress-modulating mitochondrial metabolism

Professor Win-Pin Su (left) and Professor Chih-Chia Huang (right) jointly supervised students in their research.
The principal investigator of the research team is Prof. Wen-Pin Su, who serves as the Director of the Institute of Clinical Medicine and is a member of the Center of Applied Nanomedicine at NCKU. He also serves as the Director of the Clinical Medicine Research Center at the NCKUH hospital. Due to his expertise, Prof. Su provides insights into the knowledge of anticancer drugs and tumor animal models. He guides the experimental design and execution conducted by doctoral student Li-Chan Chang at the Institute of Clinical Medicine (Dr. Chang is the first author of the paper). As for the nanoplatform, Prof. Chih-Chia Huang from the Department of Photonics and the chief of research at the Center of Applied Nanomedicine, developed this technology of polymeric nano-formulation and guide doctoral student Yu-Cheng Chin to synthesize Au@PSMA-Sit nano drug (Dr. Chin is the second author of the paper).
In NSCLCs, nearly 25% of patients carry KARS mutations, specifically at the KRAS glycine-to-cysteine substitution (G12C). Treating this subset of patients is quite challenging, with only two inhibitors entering the market in the past one to two years.
In the current clinical treatment of KRAS-mutant NSCLC, in addition to traditional chemotherapy, PD-1/PD-L1 immune therapy is often employed. However, using immune therapy alone for lung cancer treatment yields suboptimal results, with an efficacy rate of approximately 20%. To enhance therapeutic efficacy, immune therapy, such as anti-angiogenic drugs, is often combined with drugs that modulate the TME.

Professor Win-Pin Su (left) and Professor Chih-Chia Huang (right) discussed research progress in the laboratory.
Prof. Su proposed that Sitravatinib exhibits anti-angiogenic effects by inhibiting VEGFR2 and reshaping the TME through Tyro3, MerTk, Axl, and Kit receptors. However, it is currently undergoing clinical trials. Sitravatinib still faces several challenges, including its hydrophobic structure, resulting in poor entrance of the bloodstream. Consequently, therapeutic efficacy may not be achieved at low doses, while at high doses, the drug is prone to causing treatment-related side effects. Therefore, in clinical trials for NSCLCs, it is not recommended to use Sitravatinib alone. Instead, it is often combined with PD-1/PD-L1 immune therapy to improve the effectiveness of lung cancer treatment. Over the years, nanomedicines have proven to enhance the effective delivery of anticancer drugs to tumors. Therefore, the research team has decided to modify and optimize the Sitravatinib drug using nanotechnology to improve transport efficiency and reduce the risk of side effects.
Therefore, after discussions between Prof. Su and Prof. Huang, the research team selected FDA-approved materials, including PSMA polymer and gold nanoparticles. They developed an amphiphilic nanocarrier, Au@PSMA, loaded with the Sitravatinib drug, synthesizing a new nanodrug, Au@PSMA-Sit. The hydrophobic end of the PSMA polymer significantly adsorbs Sitravatinib, while the hydrophilic end enhances the drug's dispersion and stability. This configuration benefits Sitravatinib by improving its therapeutic efficacy through the enhanced permeability and retention (EPR) effect.
The research team confirmed that Au@PSMA-Sit nanodrugs exhibit outstanding antitumor efficacy in a murine KRASG12C-mutant lung cancer model, requiring a lower therapeutic dosage—only 1/10 of Sitravatinib. Without PD-1/PD-L1 immune therapy, it significantly inhibited tumor growth and metastasis. Notably, the results of the animal experiments indicate the absence of short-term toxicity.

Group photo of interdisciplinary research team members.
Utilizing the single-cell transcriptome sequencing analysis technique, Prof. Su suggested that the Au@PSMA nanoplatform not only stably delivers drugs but can also enhance the modulation of macrophage metabolism within the TME. The polarization of macrophages into the M1 type would activate T cells to boost antitumor immunity. This demonstrates the potential of nanotechnology modification and optimization, highlighting the technical prowess of the NCKU research team. With the assistance of Professor Ping-Ching Wu from the Department of Biomedical Engineering, future efforts will focus on patent deployment, aiming to validate the superior anticancer potential of Au@PSMA-Sit.
Click Num:
Share

